Back Salpetersuur Afrikaans حمض النتريك Arabic Ácidu nítrico AST Nitrat turşusu Azerbaijani نیتریک اسید AZB Азотная кіслата Byelorussian Азотавая кісьля BE-X-OLD Азотна киселина Bulgarian নাইট্রিক অ্যাসিড Bengali/Bangla Trenkenn nitrek Breton
 Pure nitric acid
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitric acid
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.832 | ||
| EC Number |
| ||
| 1576 | |||
| KEGG | |||
| MeSH | Nitric+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2031 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
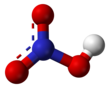
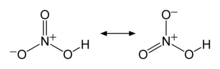
| HNO3 | |||
| Molar mass | 63.012 g·mol−1 | ||
| Appearance | Colorless liquid[1] | ||
| Odor | Acrid, suffocating[1] | ||
| Density | 1.51 g/cm3, 1.41 g/cm3 [68% w/w] | ||
| Melting point | −42 °C (−44 °F; 231 K) | ||
| Boiling point | 83 °C (181 °F; 356 K) 68% solution boils at 121 °C (250 °F; 394 K) | ||
| Miscible | |||
| log P | −0.13[2] | ||
| Vapor pressure | 48 mmHg (20 °C)[1] | ||
| Acidity (pKa) | −1.4[3] | ||
| Conjugate base | Nitrate | ||
| −1.99×10−5 cm3/mol | |||
Refractive index (nD)
|
1.397 (16.5 °C) | ||
| 2.17 ± 0.02 D | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
146 J/(mol·K)[4] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−207 kJ/mol[4] | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H272, H300, H310, H330, H373, H411 | |||
| P210, P220, P260, P305+P351+P338, P310, P370+P378 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
138 ppm (rat, 30 min)[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 2 ppm (5 mg/m3)[1] | ||
REL (Recommended)
|
TWA 2 ppm (5 mg/m3) ST 4 ppm (10 mg/m3)[1] | ||
IDLH (Immediate danger)
|
25 ppm[1] | ||
| Safety data sheet (SDS) | ICSC 0183 | ||
| Related compounds | |||
Other anions
|
Nitrous acid | ||
Other cations
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid.[6] The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agent.
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0447". National Institute for Occupational Safety and Health (NIOSH).
- ^ "nitric acid_msds".
- ^ Bell, R. P. (1973), The Proton in Chemistry (2nd ed.), Ithaca, NY: Cornell University Press
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Safety Data Sheet" (PDF). fishersci.com. Fisher Scientific International. 23 March 2015. p. 2. Archived (PDF) from the original on 10 September 2022. Retrieved 4 October 2022.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 465–471. ISBN 978-0-08-037941-8.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search