Back Stikstoftetroksied Afrikaans رباعي أكسيد ثنائي النتروجين Arabic دینیتروژن تترااوکسید AZB Диазотен тетраоксид Bulgarian Tetraòxid de dinitrogen Catalan Dinitrogentetraoxid Danish Distickstofftetroxid German Tetróxido de dinitrógeno Spanish دینیتروژن تتراکسید Persian Typpitetraoksidi Finnish
| |||
 Nitrogen dioxide at −196 °C, 0 °C, 23 °C, 35 °C, and 50 °C. (NO
2) converts to the colorless dinitrogen tetroxide (N 2O 4) at low temperatures, and reverts to NO 2 at higher temperatures. | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dinitrogen tetroxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.031.012 | ||
| EC Number |
| ||
| 2249 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1067 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| N2O4 | |||
| Molar mass | 92.010 g·mol−1 | ||
| Appearance | White solid, colorless liquid, orange gas | ||
| Density | 1.44246 g/cm3 (liquid, 21 °C) | ||
| Melting point | −11.2 °C (11.8 °F; 261.9 K) and decomposes to NO2 | ||
| Boiling point | 21.69 °C (71.04 °F; 294.84 K) | ||
| Reacts to form nitrous and nitric acids | |||
| Vapor pressure | 96 kPa (20 °C)[1] | ||
| −23.0·10−6 cm3/mol | |||
Refractive index (nD)
|
1.00112 | ||
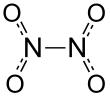
| Structure | |||
| Planar, D2h | |||
| small, non-zero | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
304.29 J/K⋅mol[2] | ||
Std enthalpy of
formation (ΔfH⦵298) |
+9.16 kJ/mol[2] | ||
| Hazards | |||
| GHS labelling: | |||
    
| |||
| Danger | |||
| H270, H280, H314, H330, H335, H336 | |||
| P220, P244, P260, P261, P264, P271, P280, P284, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P320, P321, P363, P370+P376, P403, P403+P233, P405, P410+P403, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | External SDS | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.
Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has made the pair a common bipropellant for rockets.
- ^ International Chemical Safety Card https://www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0930&p_version=2
- ^ a b P.W. Atkins and J. de Paula, Physical Chemistry (8th ed., W.H. Freeman, 2006) p.999
- ^ "Chemical Datasheet: Nitrogen tetroxide". CAMEO Chemicals NOAA. Retrieved 8 September 2020.
- ^ "Compound Summary: Dinitrogen tetroxide". PubChem. Retrieved 8 September 2020.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search