
Back Betadeeltjie Afrikaans Particla beta AN جسيم بيتا Arabic Partícula beta AST Beta hissəcik Azerbaijani Бэта-часціца Byelorussian Бета-частица Bulgarian বিটা কণিকা Bengali/Bangla Beta čestica BS Partícula β Catalan
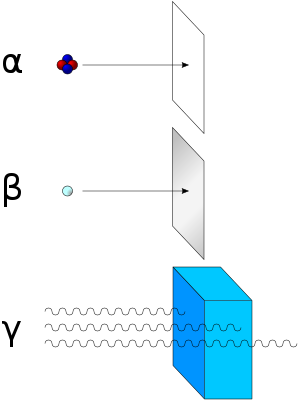
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively.[2]
Beta particles with an energy of 0.5 MeV have a range of about one metre in the air; the distance is dependent on the particle energy.
Beta particles are a type of ionizing radiation and for radiation protection purposes are regarded as being more ionising than gamma rays, but less ionising than alpha particles. The higher the ionising effect, the greater the damage to living tissue, but also the lower the penetrating power of the radiation.
- ^ "Radiation Basics". United States Nuclear Regulatory Com. 2017-10-02.
- ^ Lawrence Berkeley National Laboratory (9 August 2000). "Beta Decay". Nuclear Wall Chart. United States Department of Energy. Archived from the original on 3 March 2016. Retrieved 17 January 2016.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search