
Back Swaelsuur Afrikaans حمض الكبريتيك Arabic حمض كبريتي ARY Ácidu sulfúrico AST Sulfat turşusu Azerbaijani سولفوریک اسید AZB Серная кіслата Byelorussian Серчаная кісьля BE-X-OLD Сярна киселина Bulgarian সালফিউরিক অ্যাসিড Bengali/Bangla
| |||||||||||
 | |||||||||||
| Names | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IUPAC name
Sulfuric acid
| |||||||||||
Other names
| |||||||||||
| Identifiers | |||||||||||
3D model (JSmol)
|
|||||||||||
| ChEBI | |||||||||||
| ChEMBL | |||||||||||
| ChemSpider | |||||||||||
| ECHA InfoCard | 100.028.763 | ||||||||||
| EC Number |
| ||||||||||
| E number | E513 (acidity regulators, ...) | ||||||||||
| 2122 | |||||||||||
| KEGG | |||||||||||
PubChem CID
|
|||||||||||
| RTECS number |
| ||||||||||
| UNII | |||||||||||
| UN number | 1830 | ||||||||||
CompTox Dashboard (EPA)
|
|||||||||||
| |||||||||||
| |||||||||||
| Properties | |||||||||||
| H2SO4, sometimes expressed (HO)2SO2 | |||||||||||
| Molar mass | 98.079 g/mol | ||||||||||
| Appearance | Colorless viscous liquid | ||||||||||
| Odor | Odorless | ||||||||||
| Density | 1.8302 g/cm3, liquid[1] | ||||||||||
| Melting point | 10.31[1] °C (50.56 °F; 283.46 K) | ||||||||||
| Boiling point | 337[1] °C (639 °F; 610 K) When sulfuric acid is above 300 °C (572 °F; 573 K), it gradually decomposes to SO3 + H2O | ||||||||||
| miscible, exothermic | |||||||||||
| Vapor pressure | 0.001 mmHg (20 °C)[2] | ||||||||||
| Acidity (pKa) | pKa1 = −2.8 pKa2 = 1.99 | ||||||||||
| Conjugate base | Bisulfate | ||||||||||
| Viscosity | 26.7 cP (20 °C) | ||||||||||
| Structure[3] | |||||||||||
| monoclinic | |||||||||||
| C2/c | |||||||||||
a = 818.1(2) pm, b = 469.60(10) pm, c = 856.3(2) pm α = 90°, β = 111.39(3)
°, γ = 90° | |||||||||||
Formula units (Z)
|
4 | ||||||||||
| Thermochemistry | |||||||||||
Std molar
entropy (S⦵298) |
157 J/(mol·K)[4] | ||||||||||
Std enthalpy of
formation (ΔfH⦵298) |
−814 kJ/mol[4] | ||||||||||
Enthalpy of vaporization (ΔfHvap)
|
56 kJ/mol[5] | ||||||||||
| Hazards | |||||||||||
| GHS labelling: | |||||||||||
 
| |||||||||||
| Danger | |||||||||||
| H314 | |||||||||||
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |||||||||||
| NFPA 704 (fire diamond) | |||||||||||
| Flash point | Non-flammable | ||||||||||
Threshold limit value (TLV)
|
15 mg/m3 (IDLH), 1 mg/m3 (TWA), 2 mg/m3 (STEL) | ||||||||||
| Lethal dose or concentration (LD, LC): | |||||||||||
LD50 (median dose)
|
2140 mg/kg (rat, oral)[6] | ||||||||||
LC50 (median concentration)
|
| ||||||||||
LCLo (lowest published)
|
87 mg/m3 (guinea pig, 2.75 hr)[6] | ||||||||||
| NIOSH (US health exposure limits): | |||||||||||
PEL (Permissible)
|
TWA 1 mg/m3[2] | ||||||||||
REL (Recommended)
|
TWA 1 mg/m3[2] | ||||||||||
IDLH (Immediate danger)
|
15 mg/m3[2] | ||||||||||
| Related compounds | |||||||||||
Related strong acids
|
|||||||||||
Related compounds
|
|||||||||||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||||||||||
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.[7]
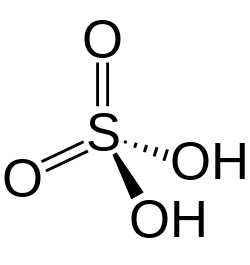
Pure sulfuric acid does not occur naturally due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air.[7] Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus, the reverse procedure of adding water to the acid is generally avoided since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic chemical burns and secondary thermal burns due to dehydration.[8][9] Dilute sulfuric acid is substantially less hazardous without the oxidative and dehydrating properties; though, it is handled with care for its acidity.
Many methods for its production are known, including the contact process, the wet sulfuric acid process, and the lead chamber process.[10] Sulfuric acid is also a key substance in the chemical industry. It is most commonly used in fertilizer manufacture[11] but is also important in mineral processing, oil refining, wastewater treating, and chemical synthesis. It has a wide range of end applications, including in domestic acidic drain cleaners,[12] as an electrolyte in lead-acid batteries, as a dehydrating compound, and in various cleaning agents. Sulfuric acid can be obtained by dissolving sulfur trioxide in water.
- ^ a b c Haynes, William M. (2014). CRC Handbook of Chemistry and Physics (95 ed.). CRC Press. pp. 4–85. ISBN 9781482208689. Retrieved 18 November 2018.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0577". National Institute for Occupational Safety and Health (NIOSH).
- ^ Kemnitz, E.; Werner, C.; Trojanov, S. (15 November 1996). "Reinvestigation of Crystalline Sulfuric Acid and Oxonium Hydrogensulfate". Acta Crystallographica Section C Crystal Structure Communications. 52 (11): 2665–2668. Bibcode:1996AcCrC..52.2665K. doi:10.1107/S0108270196006749.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ^ "PubChem Compound Summary for CID 1118, Sulfuric Acid". PubChem. National Center for Biotechnology Information. Retrieved 14 January 2025. 3.2.17 Heat of Vaporization.
- ^ a b c "Sulfuric acid". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Sulfuric acid safety data sheet" (PDF). arkema-inc.com. Archived from the original (PDF) on 17 June 2012.
Clear to turbid oily odorless liquid, colorless to slightly yellow.
- ^ Cite error: The named reference
OAwas invoked but never defined (see the help page). - ^ "BASF Chemical Emergency Medical Guidelines – Sulfuric acid (H2SO4)" (PDF). BASF Chemical Company. 2012. Archived from the original (PDF) on 14 June 2019. Retrieved 18 December 2014.
- ^ Hermann Müller "Sulfuric Acid and Sulfur Trioxide" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. 2000 doi:10.1002/14356007.a25_635
- ^ "Sulfuric acid". essentialchemicalindustry.org.
- ^ Cite error: The named reference
dcwas invoked but never defined (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search


