
Back Kwik(I)chloried Afrikaans جیوه (I) کولورید AZB Chlorid rtuťný Czech Чĕркĕмĕл(I) хлори(д)чĕ CV Quecksilber(I)-chlorid German Χλωριούχος υφυδράργυρος Greek Cloruro de mercurio(I) Spanish جیوه (I) کلرید Persian Elohopea(I)kloridi Finnish Chlorure de mercure(I) French

| |
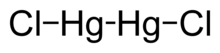
| |

| |
| Names | |
|---|---|
| IUPAC name
Dimercury dichloride
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.266 |
| EC Number |
|
| 25976 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Hg2Cl2 | |
| Molar mass | 472.09 g/mol |
| Appearance | White solid |
| Density | 7.150 g/cm3 |
| Melting point | 383 °C (721 °F; 656 K) (sublimes) |
| 0.2 mg/100 mL | |
Solubility product (Ksp)
|
1.43×10−18[1] |
| Solubility | insoluble in ethanol, ether |
| −26.0·10−6 cm3/mol | |
Refractive index (nD)
|
1.973 |
| Structure | |
| tetragonal | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
196 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−265 kJ·mol−1[2] |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H315, H319, H335, H410 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
210 mg/kg (rat, oral)[3] |
| Safety data sheet (SDS) | ICSC 0984 |
| Related compounds | |
Other anions
|
Mercury(I) fluoride Mercury(I) bromide Mercury(I) iodide |
Related compounds
|
Mercury(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel[4] (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. It is a component of reference electrodes in electrochemistry.[5][6]
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Mercury compounds [except (organo) alkyls] (as Hg)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- ^ Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. pp. 696–697. ISBN 978-0-13-039913-7.
- ^ Skoog, Douglas A.; Holler, F. James; Nieman, Timothy A. (1998). Principles of Instrumental Analysis (5th ed.). Saunders College Pub. pp. 253–271. ISBN 978-0-03-002078-0.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
