
Back Wolframaat Afrikaans Volframat BS Wolframate German Wolframato Spanish Tungstate French Wolframat ID Вольфраматтар Kazakh 중석 Korean Вольфраматтар Kirghiz Wolframaat Dutch
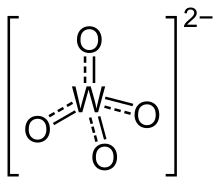
In chemistry, a tungstate is a compound that contains an oxyanion of tungsten or is a mixed oxide containing tungsten. The simplest tungstate ion is WO2−4, "orthotungstate".[1] Many other tungstates belong to a large group of polyatomic ions that are termed polyoxometalates, ("POMs"), and specifically termed isopolyoxometalates as they contain, along with oxygen and maybe hydrogen, only one other element. Almost all useful tungsten ores are tungstates.[2]
- ^ Egon Wiberg, Arnold Frederick Holleman (2001). Inorganic Chemistry. Elsevier. ISBN 0-12-352651-5.
- ^ Lassner, Erik; Schubert, Wolf-Dieter; Lüderitz, Eberhard; Wolf, Hans Uwe (2005). "Tungsten, Tungsten Alloys, and Tungsten Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_229. ISBN 978-3527306732.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search