
Back سماحية Arabic প্ৰৱেশ্যতা Assamese Дыэлектрычная пранікальнасць Byelorussian Дыэлектрычная пранікальнасьць BE-X-OLD Диелектрична проницаемост Bulgarian Permitivitat Catalan Permitivita Czech Permittivitet Danish Permittivität German Permitividad Spanish
| Articles about |
| Electromagnetism |
|---|
 |
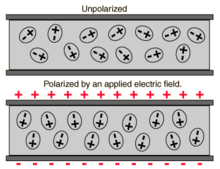
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter ε (epsilon), is a measure of the electric polarizability of a dielectric material. A material with high permittivity polarizes more in response to an applied electric field than a material with low permittivity, thereby storing more energy in the material. In electrostatics, the permittivity plays an important role in determining the capacitance of a capacitor.
In the simplest case, the electric displacement field D resulting from an applied electric field E is
More generally, the permittivity is a thermodynamic function of state.[1] It can depend on the frequency, magnitude, and direction of the applied field. The SI unit for permittivity is farad per meter (F/m).
The permittivity is often represented by the relative permittivity εr which is the ratio of the absolute permittivity ε and the vacuum permittivity εo
This dimensionless quantity is also often and ambiguously referred to as the permittivity. Another common term encountered for both absolute and relative permittivity is the dielectric constant which has been deprecated in physics and engineering[2] as well as in chemistry.[3]
By definition, a perfect vacuum has a relative permittivity of exactly 1 whereas at standard temperature and pressure, air has a relative permittivity of εr air ≡ κair ≈ 1.0006 .
Relative permittivity is directly related to electric susceptibility (χ) by
otherwise written as
The term "permittivity" was introduced in the 1880s by Oliver Heaviside to complement Thomson's (1872) "permeability".[4][irrelevant citation] Formerly written as p, the designation with ε has been in common use since the 1950s.
- ^ Landau, L. D.; Lifshitz, E. M.; Pitaevskii, L. P. (2009). Electrodynamics of continuous media. Elsevier Butterworth-Heinemann. ISBN 978-0-7506-2634-7. OCLC 756385298.
- ^ IEEE Standard Definitions of Terms for Radio Wave Propagation (Report). IEEE. 1997. p. 6. IEEE STD 211-1997.
- ^ Braslavsky, S.E. (2007). "Glossary of terms used in photochemistry (IUPAC recommendations 2006)" (PDF). Pure and Applied Chemistry. 79 (3): 293–465. doi:10.1351/pac200779030293. S2CID 96601716.
- ^ Fleming, John Ambrose (1910). The Principles of Electric Wave Telegraphy. p. 340.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search



