
Back ميمانتين Arabic ممانتین AZB Memantin Czech Memantin Danish Memantin German Μεμαντίνη Greek Memantina Spanish ممانتین Persian Memantiini Finnish Mémantine French
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Axura, Ebixa, Namenda, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604006 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | NMDA receptor antagonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Liver (<10%) |
| Elimination half-life | 60–100 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.937 |
| Chemical and physical data | |
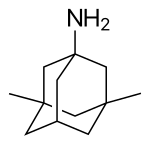
| Formula | C12H21N |
| Molar mass | 179.307 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Memantine, sold under the brand name Axura among others, is a medication used to slow the progression of moderate-to-severe Alzheimer's disease.[7][8] It is taken by mouth.[7]
Common side effects include headache, constipation, sleepiness, and dizziness.[7][8] Severe side effects may include blood clots, psychosis, and heart failure.[8] It is believed to work by acting on NMDA receptors, working as pore blockers of these ion channels.[7]
Memantine was approved for medical use in the United States in 2003.[7] It is available as a generic medication.[8] In 2021, it was the 170th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[9][10]
- ^ Cite error: The named reference
brandswas invoked but never defined (see the help page). - ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Ebixa 10 mg film-coated tablets Summary of Product Characteristics (SmPC)". (emc). 13 December 2021. Retrieved 2 May 2024.
- ^ "Namenda- memantine hydrochloride tablet; Namenda- memantine hydrochloride kit". DailyMed. 1 November 2018. Retrieved 2 May 2024.
- ^ "Namenda XR- memantine hydrochloride capsule, extended release; Namenda XR- memantine hydrochloride kit". DailyMed. 15 November 2019. Retrieved 2 May 2024.
- ^ "Axura EPAR". European Medicines Agency (EMA). 17 May 2002. Retrieved 27 February 2024.
- ^ a b c d e "Memantine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 25 April 2019. Retrieved 3 March 2019.
- ^ a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 303–304. ISBN 9780857113382.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Memantine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search