
Back بنية الألماس المكعبة Arabic Diamantstruktur German ساختار الماس Persian Timanttirakenne Finnish Structure diamant French Структура алмазу Ukrainian 鑽石結構 Chinese
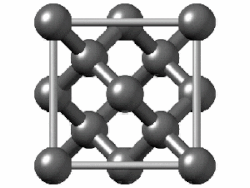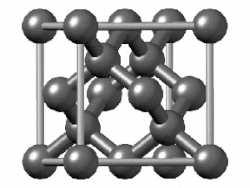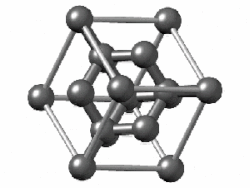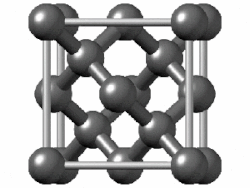


In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the semiconductors silicon and germanium, and silicon–germanium alloys in any proportion. There are also crystals, such as the high-temperature form of cristobalite, which have a similar structure, with one kind of atom (such as silicon in cristobalite) at the positions of carbon atoms in diamond but with another kind of atom (such as oxygen) halfway between those (see Category:Minerals in space group 227).
Although often called the diamond lattice, this structure is not a lattice in the technical sense of this word used in mathematics.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search