
Back عدد كم مداري Arabic Орбитално квантово число Bulgarian সহকারী কোয়ান্টাম সংখ্যা Bengali/Bangla Niver pementadel eilrenk Breton Azimutalni kvantni broj BS Nombre quàntic azimutal Catalan Vedlejší kvantové číslo Czech Nebenquantenzahl German Αζιμουθιακός κβαντικός αριθμός Greek Número cuántico azimutal Spanish
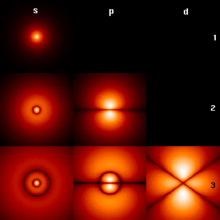
In quantum mechanics, the azimuthal quantum number ℓ is a quantum number for an atomic orbital that determines its orbital angular momentum and describes aspects of the angular shape of the orbital. The azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron (the others being the principal quantum number n, the magnetic quantum number mℓ, and the spin quantum number ms).
For a given value of the principal quantum number n (electron shell), the possible values of ℓ are the integers from 0 to n − 1. For instance, the n = 1 shell has only orbitals with , and the n = 2 shell has only orbitals with , and .
For a given value of the azimuthal quantum number ℓ, the possible values of the magnetic quantum number mℓ are the integers from mℓ=-ℓ to mℓ=+ℓ, including 0. In addition, the spin quantum number ms can take two distinct values. The set of orbitals associated with a particular value of ℓ are sometimes collectively called a subshell.
While originally used just for isolated atoms, atomic-like orbitals play a key role in the configuration of electrons in compounds including gases, liquids and solids. The quantum number ℓ plays an important role here via the connection to the angular dependence of the spherical harmonics for the different orbitals around each atom.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search

