
Back فنلافاكسين Arabic ونلافاوکسین AZB Fenlaffacsin Welsh Venlafaxin German Βενλαφαξίνη Greek Venlafaxina Spanish ونلافاکسین Persian Venlafaksiini Finnish Venlafaxine French ונלפאקסין HE
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɛnləˈfæksiːn/ VEN-lə-FAK-seen |
| Trade names | Effexor, Efexor, Venbysi XR, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694020 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| Drug class | Serotonin–norepinephrine reuptake inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 42±15%[2] |
| Protein binding | 27±2% (parent compound), 30±12% (active metabolite, desvenlafaxine)[6] |
| Metabolism | Extensively metabolised by the liver,[2][6] primarily via CYP2D6[8] |
| Metabolites | O-desmethylvenlafaxine (ODV), see desvenlafaxine |
| Elimination half-life | 5±2 h (parent compound for immediate release preparations), 15±6 h (parent compound for extended release preparations), 11±2 h (active metabolite)[2][6] |
| Excretion | Kidney (87%; 5% as unchanged drug; 29% as desvenlafaxine and 53% as other metabolites)[2][6] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.122.418 |
| Chemical and physical data | |
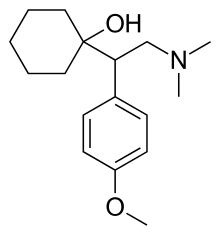
| Formula | C17H27NO2 |
| Molar mass | 277.408 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Venlafaxine, sold under the brand name Effexor among others, is an antidepressant medication of the serotonin–norepinephrine reuptake inhibitor (SNRI) class.[6][9] It is used to treat major depressive disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder.[9] Studies have shown that venlafaxine improves post-traumatic stress disorder (PTSD).[10] It may also be used for chronic pain.[11] It is taken orally (swallowed by mouth).[9] It is also available as the salt venlafaxine besylate (venlafaxine benzenesulfonate monohydrate) in an extended-release formulation (Venbysi XR).[7]
Common side effects include loss of appetite, constipation, dry mouth, dizziness, sweating, insomnia, drowsiness and sexual problems.[9] Severe side effects include an increased risk of suicide, mania, and serotonin syndrome.[9] Antidepressant withdrawal syndrome may occur if stopped.[9] There are concerns that use during the later part of pregnancy can harm the baby.[9] How it works is not entirely clear, but it seems to be related to the potentiation of the activity of some neurotransmitters in the brain.[9]
Venlafaxine was approved for medical use in the United States in 1993.[9] It is available as a generic medication.[9] In 2021, it was the 44th most commonly prescribed medication in the United States with more than 15 million prescriptions.[12][13]
- ^ Cite error: The named reference
brandswas invoked but never defined (see the help page). - ^ a b c d e f Cite error: The named reference
TGAwas invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Efexor XL 75 mg hard prolonged release capsules - Summary of Product Characteristics (SmPC)". (emc). 16 March 2020. Archived from the original on 7 October 2020. Retrieved 15 April 2020.
- ^ a b c d e f "Effexor XR- venlafaxine hydrochloride capsule, extended release". DailyMed. Archived from the original on 12 May 2021. Retrieved 11 May 2021.
- ^ a b "Venlafaxine tablet, extended release". DailyMed. 30 June 2022. Retrieved 7 January 2023.
- ^ Dean L (2015). Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kane MS, Kattman BL, Malheiro AJ (eds.). Venlafaxine Therapy and CYP2D6 Genotype. National Center for Biotechnology Information (US). PMID 28520361. Archived from the original on 29 November 2017. Retrieved 28 December 2018.
- ^ a b c d e f g h i j "Venlafaxine Hydrochloride Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 27 November 2020. Retrieved 24 December 2018.
- ^ Forman-Hoffman V, Middleton JC, Feltner C, Gaynes BN, Weber RP, Bann C, et al. (17 May 2018). Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update (Report). Agency for Healthcare Research and Quality (AHRQ). doi:10.23970/ahrqepccer207 (inactive 4 March 2024). PMID 30204376. Archived from the original on 10 July 2018. Retrieved 29 July 2023.
{{cite report}}: CS1 maint: DOI inactive as of March 2024 (link) - ^ "Antidepressants: Another weapon against chronic pain". Mayo Clinic. Archived from the original on 26 October 2021. Retrieved 25 January 2020.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Venlafaxine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search