Spectroelectrochemistry (SEC) is a set of multi-response analytical techniques in which complementary chemical information (electrochemical and spectroscopic) is obtained in a single experiment. Spectroelectrochemistry provides a whole vision of the phenomena that take place in the electrode process.[1][2][3][4][5] The first spectroelectrochemical experiment was carried out by Theodore Kuwana, PhD, in 1964.[6]
The main objective of spectroelectrochemical experiments is to obtain simultaneous, time-resolved and in-situ electrochemical and spectroscopic information on reactions taking place on the electrode surface.[1] The base of the technique consist in studying the interaction of a beam of electromagnetic radiation with the compounds involved in these reactions. The changes of the optical and electrical signal allow us to understand the evolution of the electrode process.
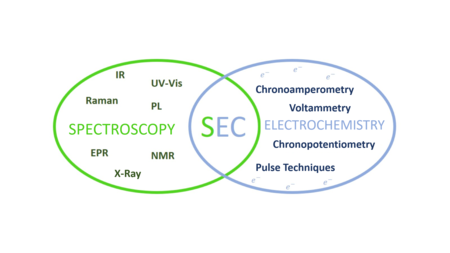
The techniques on which the spectroelectrochemistry is based are:
- Electrochemistry, which studies the interaction between electrical energy and chemical changes. This technique allows us to analyse reactions that involve electron transfer processes (redox reactions).[7]
- Spectroscopy, which studies the interaction between electromagnetic radiation and matter (absorption, dispersion or emission).[8][9]
Spectroelectrochemistry provides molecular, thermodynamic and kinetic information of reagents, products and/or intermediates involved in the electron transfer process.[1][2][3][4][5]
- ^ a b c Garoz‐Ruiz, Jesus; Perales‐Rondon, Juan Victor; Heras, Aranzazu; Colina, Alvaro (5 April 2019). "Spectroelectrochemical Sensing: Current Trends and Challenges". Electroanalysis. 31 (7): 1254–1278. doi:10.1002/elan.201900075. hdl:10259/6122. S2CID 133304199.
- ^ a b Keyes, Tia E.; Forster, Robert J. (2007). Handbook of electrochemistry (1st ed.). Amsterdam, Netherlands: Elsevier. ISBN 9780444519580.
- ^ a b Kaim, Wolfgang; Fiedler, Jan (2009). "Spectroelectrochemistry: the best of two worlds". Chemical Society Reviews. 38 (12): 3373–3382. doi:10.1039/b504286k. PMID 20449056.
- ^ a b Lozeman, Jasper J. A.; Führer, Pascal; Olthuis, Wouter; Odijk, Mathieu (2020). "Spectroelectrochemistry, the future of visualizing electrode processes by hyphenating electrochemistry with spectroscopic techniques". The Analyst. 145 (7): 2482–2509. Bibcode:2020Ana...145.2482L. doi:10.1039/c9an02105a. PMID 31998878.
- ^ a b Zhai, Yanling; Zhu, Zhijun; Zhou, Susan; Zhu, Chengzhou; Dong, Shaojun (2018). "Recent advances in spectroelectrochemistry". Nanoscale. 10 (7): 3089–3111. doi:10.1039/c7nr07803j. PMID 29379916.
- ^ Kuwana, Theodore.; Darlington, R. K.; Leedy, D. W. (September 1964). "Electrochemical Studies Using Conducting Glass Indicator Electrodes". Analytical Chemistry. 36 (10): 2023–2025. doi:10.1021/ac60216a003.
- ^ Elgrishi, Noémie; Rountree, Kelley J.; McCarthy, Brian D.; Rountree, Eric S.; Eisenhart, Thomas T.; Dempsey, Jillian L. (3 November 2017). "A Practical Beginner's Guide to Cyclic Voltammetry". Journal of Chemical Education. 95 (2): 197–206. doi:10.1021/acs.jchemed.7b00361.
- ^ Braun, Robert D. (2006). Introduction to instrumental analysis (5th ed.). New York, United States: W.H. Freeman & Co Ltd. ISBN 978-8188449156.
- ^ Skoog, Douglas; Holler, James F; Crouch, Stanley (2001). Principios de análisis instrumental (6 ed.). México: CENCAGE Learning. pp. 481–498. ISBN 9788578110796.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
