Back Natriumbikarbonaat Afrikaans بيكربونات الصوديوم Arabic Bicarbonatu de sodiu AST Natrium-hidrokarbonat Azerbaijani بیکربونات سودیوم AZB Натриев бикарбонат Bulgarian সোডিয়াম বাইকার্বনেট Bengali/Bangla Natrij-hidrogenkarbonat BS Hidrogencarbonat de sodi Catalan Hydrogenuhličitan sodný Czech
| |||
| |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
sodium hydrogencarbonate
| |||
| Other names
Baking soda, bicarb (laboratory slang), bicarbonate of soda, nahcolite, natrium hydrogen carbonate, natron
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 4153970 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.122 | ||
| EC Number |
| ||
| E number | E500(ii) (acidity regulators, ...) | ||
| KEGG | |||
| MeSH | Sodium+bicarbonate | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
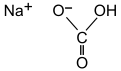
| NaHCO 3 | |||
| Molar mass | 84.0066 g mol−1 | ||
| Appearance | White crystals | ||
| Odor | Odorless | ||
| Density |
| ||
| Melting point | (Decomposes to sodium carbonate starting at 50 °C[1][6]) | ||
| Solubility | 0.02 wt% acetone, 2.13 wt% methanol @22 °C.[4] insoluble in ethanol | ||
| log P | −0.82 | ||
| Acidity (pKa) | |||
Refractive index (nD)
|
nα = 1.377 nβ = 1.501 nγ = 1.583 | ||
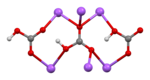
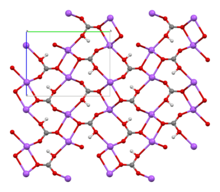
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
Heat capacity (C)
|
87.6 J/mol K[7] | ||
Std molar
entropy (S⦵298) |
101.7 J/mol K[7] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−950.8 kJ/mol[7] | ||
Gibbs free energy (ΔfG⦵)
|
−851.0 kJ/mol[7] | ||
| Pharmacology | |||
| B05CB04 (WHO) B05XA02 (WHO), QG04BQ01 (WHO) | |||
| Intravenous, oral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Causes serious eye irritation | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Incombustible | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
4220 mg/kg (rat, oral)[8] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions
|
Sodium carbonate | ||
Other cations
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate[9]), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3−). Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite, although it is more commonly found as a component of the mineral trona.[10]
Because it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, brewing soda and bicarbonate of soda and can often be found near baking powder in stores. The term baking soda is more common in the United States, while bicarbonate of soda is more common in Australia, the United Kingdom, and New Zealand.[11] Abbreviated colloquial forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common.[12]
Its E number food additive code is E500.[13]
The prefix bi- in "bicarbonate" comes from an outdated naming system predating molecular knowledge. It is based on the observation that there is twice as much carbonate (CO3−2) per sodium in sodium bicarbonate (NaHCO3) as there is in sodium carbonate (Na2CO3).[14] The modern chemical formulas of these compounds now express their precise chemical compositions which were unknown when the name bi-carbonate of potash was coined (see also: bicarbonate).
- ^ a b Haynes, p. 4.90
- ^ a b c Haynes, p. 5.194
- ^ a b c "Sodium Bicarbonate" (PDF). United Nations Environment Programme. Archived from the original (PDF) on 16 May 2011.
- ^ Ellingboe JL, Runnels JH (1966). "Solubilities of Sodium Carbonate and Sodium Bicarbonate in Acetone-Water and Methanol-Water Mixtures". J. Chem. Eng. Data. 11 (3): 323–324. doi:10.1021/je60030a009.
- ^ a b Haynes, p. 7.23
- ^ Pasquali I, Bettini R, Giordano F (2007). "Thermal behaviour of diclofenac, diclofenac sodium and sodium bicarbonate compositions". Journal of Thermal Analysis and Calorimetry. 90 (3): 903–907. doi:10.1007/s10973-006-8182-1. S2CID 95695262.
- ^ a b c d Haynes, p. 5.19
- ^ Griffith JF (1964). "Interlaboratory variations in the determination of acute oral LD50". Toxicology and Applied Pharmacology. 6 (6): 726–730. doi:10.1016/0041-008X(64)90124-3. PMID 14235840.
- ^ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (PDF), IUPAC, p. 137, archived (PDF) from the original on 18 May 2017
- ^ Gärtner RS, Witkamp GJ (August 2007). "Mixed solvent reactive recrystallization of trona (sodium sesqui-carbonate) into soda (sodium carbonate anhydrate)". Hydrometallurgy. 88 (1–4): 75–91. doi:10.1016/j.hydromet.2007.03.006.
- ^ "Baking powder, baking soda or bicarbonate of soda?". Reader's Digest Australia. Retrieved 2 June 2024.
- ^ PubChem. "Sodium bicarbonate". pubchem.ncbi.nlm.nih.gov. Retrieved 25 January 2021.
- ^ "Approved additives and E numbers". Food Standards Agency. Retrieved 7 December 2020.
- ^ Wollaston WH (January 1814). "I. A Synoptic scale of chemical equivalents". Philosophical Transactions of the Royal Society of London. 104: 1–22. doi:10.1098/rstl.1814.0001. S2CID 96774986.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search