
Back سيلينيت (أيون) Arabic Selenito Spanish Seleniitti Finnish Sélénite (ion) French Selenito Italian Селениты (соли) Russian Selenit (jon) Serbo-Croatian Selenite SIMPLE Selenit (jon) Serbian செலீனைட்டு Tamil
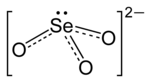
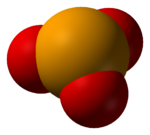

Selenite refers to the anion with the chemical formula SeO2−3. It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, SO2−3. Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite Na2SeO3 which is a common source of selenite.[1] Selenite also refers to the esters of selenous acid, for example dimethyl selenite (CH3)2SeO3.
- ^ F. Fehér (1963). "Sodium Selenite (IV)". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1pages=431. NY, NY: Academic Press.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search