Back حمض الساليسيليك Arabic اسید سالیسیلیک AZB Саліцылавая кіслата Byelorussian Салицилова киселина Bulgarian Salicilna kiselina BS Àcid salicílic Catalan Kyselina salicylová Czech Asid salicylig Welsh Salicylsyre Danish Salicylsäure German
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
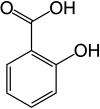
2-Hydroxybenzoic acid[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.648 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.122 g/mol | ||
| Appearance | Colorless to white crystals | ||
| Odor | Odorless | ||
| Density | 1.443 g/cm3 (20 °C)[2] | ||
| Melting point | 158.6 °C (317.5 °F; 431.8 K) | ||
| Boiling point | 211 °C (412 °F; 484 K) at 20 mmHg[2][3] | ||
| Sublimes at 76 °C[3] | |||
| Solubility | Soluble in ether, CCl4, benzene, propanol, acetone, ethanol, oil of turpentine, toluene | ||
| Solubility in benzene | |||
| Solubility in chloroform | |||
| Solubility in methanol |
| ||
| Solubility in olive oil | 2.43 g/100 g (23 °C)[3] | ||
| Solubility in acetone | 39.6 g/100 g (23 °C)[3] | ||
| log P | 2.26 | ||
| Vapor pressure | 10.93 mPa[3] | ||
| Acidity (pKa) | |||
| UV-vis (λmax) | 210 nm, 234 nm, 303 nm (4 mg/dL in ethanol)[3] | ||
| −72.23·10−6 cm3/mol | |||
Refractive index (nD)
|
1.565 (20 °C)[2] | ||
| 2.65 D | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
−589.9 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
-3.025 MJ/mol[6] | ||
| Pharmacology | |||
| A01AD05 (WHO) B01AC06 (WHO) D01AE12 (WHO) N02BA01 (WHO) S01BC08 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Eye hazards
|
Severe irritation | ||
Skin hazards
|
Mild irritation | ||
| GHS labelling:[7] | |||
 
| |||
| Danger | |||
| H302, H318 | |||
| P280, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 157 °C (315 °F; 430 K) closed cup[3] | ||
| 540 °C (1,004 °F; 813 K)[3] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
480 mg/kg (mice, oral) | ||
| Safety data sheet (SDS) | MSDS[dead link] | ||
| Related compounds | |||
Related compounds
|
Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid, Salicylate synthase | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Salicylic acid is an organic compound with the formula HOC6H4COOH.[3] A colorless (or, white), bitter-tasting solid, it is a precursor to and a metabolite of acetylsalicylic acid (aspirin).[3] It is a plant hormone,[8] and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen.[9] The name is from Latin salix for willow tree, from which it was initially identified and derived. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates.[3]
- ^ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 64. doi:10.1039/9781849733069-FP001 (inactive 2024-04-11). ISBN 978-0-85404-182-4.
{{cite book}}: CS1 maint: DOI inactive as of April 2024 (link) - ^ a b c Haynes WM, ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.306. ISBN 1-4398-5511-0.
- ^ a b c d e f g h i j k l m n o p "Salicylic acid". PubChem, US National Library of Medicine. 19 Nov 2023. Retrieved 19 Nov 2023.
- ^ a b c Atherton Seidell, William F. Linke (1952). Solubilities of Inorganic and Organic Compounds: A Compilation of Solubility Data from the Periodical Literature. Supplement to the third edition containing data published during the years 1939–1949. Van Nostrand.
- ^ Wishart DS, Djombou Feunang Y, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. "Salycylic acid | DrugBank Online". DrugBank. 5.0.
- ^ "Salicylic acid". Archived from the original on 2017-02-15. Retrieved 2014-08-17.
- ^ Sigma-Aldrich Co., Salicylic acid.
- ^ Boullard O, Leblanc H, Besson B (2000). "Salicylic Acid". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a23_477. ISBN 3-527-30673-0.
- ^ Lewis Sr RJ (2008). Hazardous Chemicals Desk Reference. John Wiley & Sons. p. 1217. ISBN 978-0-470-33445-4.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search