
Back فينانثرين Arabic Fenantren Azerbaijani فنانترن AZB Фенантрэн Byelorussian Fenantrè Catalan Fenanthren Czech Phenanthren German Φαινανθρένιο Greek Fenantreno Esperanto Fenantreno Spanish
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Phenanthrene | |
| Identifiers | |
3D model (JSmol)
|
|
| 1905428 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.437 |
| EC Number |
|
| 28699 | |
| KEGG | |
| MeSH | C031181 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H10 | |
| Molar mass | 178.234 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.18 g/cm3[1] |
| Melting point | 101 °C (214 °F; 374 K)[1] |
| Boiling point | 332 °C (630 °F; 605 K)[1] |
| 1.6 mg/L[1] | |
| −127.9·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 171 °C (340 °F; 444 K)[1] |
| Structure | |
| C2v[2] | |
| 0 D | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
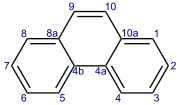
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, explosives, and drugs. It has also been used to make bile acids, cholesterol and steroids.[3]
Phenanthrene occurs naturally and also is a man-made chemical. Commonly, humans are exposed to phenanthrene through inhalation of cigarette smoke, but there are many routes of exposure. Animal studies have shown that phenanthrene is a potential carcinogen.[3] However, according to IARC, it is not identified as a probable, possible or confirmed human carcinogen.[4]
Phenanthrene's three fused rings are angled as in the phenacenes, rather than straight as in the acenes. The compound with a phenanthrene skeleton and nitrogens at the 4 and 5 positions is known as phenanthroline.
- ^ a b c d e Record of CAS RN 85-01-8 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Peter Atkins, J. D. P., Atkins' Physical Chemistry. Oxford: 2010. P. 443.
- ^ a b "Phenanthrene Fact Sheet" (PDF). archive.epa.gov. U.S. Environmental Protection Agency. Retrieved 19 July 2019.
- ^ "Phenanthrene". Sigma-Alrdich.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
