
Back Perfluortributylamin Czech Perfluortributylamin German Perfluorotributylamine French Perfluortributil-amin Hungarian Perfluortributylamine Dutch 全氟三丁胺 Chinese
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
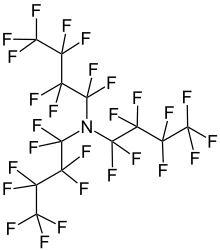
1,1,2,2,3,3,4,4,4-Nonafluoro-N,N-bis(nonafluorobutyl)butan-1-amine | |
| Other names
Fluorinert
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | PFTBA |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.659 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| N(CF2CF2CF2CF3)3 | |
| Molar mass | 671.096 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.884 g/mL |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 178 °C (352 °F; 451 K) |
| Insoluble | |
| Solubility in methanol and isopropyl alcohol | Insoluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perfluorotributylamine (PFTBA), also referred to as FC43, is an organic compound with the chemical formula N(CF2CF2CF2CF3)3. It is a colorless liquid. A molecule of this chemical compound consists of three butyl groups connected to one nitrogen atom, in which all of the hydrogen atoms are replaced with fluorine atoms. The compound is produced for the electronics industry, along with other perfluoroalkylamines. The high degree of fluorination significantly reduces the basicity of the central amine due to electron-withdrawing effects.[1]
- ^ "Tuning basicity | Cambridge MedChem Consulting". www.cambridgemedchemconsulting.com. Retrieved 2020-08-11.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search