Back اوکسالیل کولورید AZB Clorur d'oxalil Catalan Oxalylchlorid Czech Oxalylchlorid German Oksalila klorido Esperanto اکسالیل کلرید Persian Oksalyylikloridi Finnish Chlorure d'oxalyle French ऑक्जिलिल क्लोराइड Hindi Oxalil-klorid Hungarian

| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalyl dichloride[1] | |||
| Systematic IUPAC name
Ethanedioyl dichloride[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.092 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
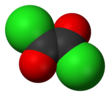
| C2O2Cl2 | |||
| Molar mass | 126.92 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Phosgene-like[2] | ||
| Density | 1.4785 g/mL | ||
| Melting point | −16 °C (3 °F; 257 K) | ||
| Boiling point | 63 to 64 °C (145 to 147 °F; 336 to 337 K) at 1.017 bar | ||
| Reacts | |||
Refractive index (nD)
|
1.429 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, corrosive, lachrymator[3] | ||
| GHS labelling: | |||
  [3] [3]
| |||
| Danger[3] | |||
| H314, H331[3] | |||
| P261, P280, P305+P351+P338, P310[3] | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related acyl chlorides
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Oxalyl chloride is an organic chemical compound with the formula Cl−C(=O)−C(=O)−Cl. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.[4]
- ^ a b Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 797. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Oxalyl chloride: odor
- ^ a b c d e Oxalyl chloride MSDS
- ^ Salmon, R. (2001). "Oxalyl Chloride". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.ro015. ISBN 0471936235.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search