
Back أميد الليثيوم Arabic Amid lithný Czech Lithiumamid German Λιθιαμίδιο Greek Amida de litio Spanish لیتیم آمید Persian Litiumamidi Finnish Amidure de lithium French लिथियम एमाइड Hindi Litium amida ID
 | |
| Names | |
|---|---|
| IUPAC name
Lithium amide
| |
| Other names
Lithium azanide
Lithamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.062 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LiNH2 | |
| Molar mass | 22.96 g·mol−1 |
| Appearance | white solid |
| Density | 1.178 g/cm3 |
| Melting point | 375 °C (707 °F; 648 K) |
| Boiling point | 430 °C (806 °F; 703 K) decomposes |
| reacts | |
| Solubility | slightly soluble in ethanol insoluble in ammonia |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-182 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
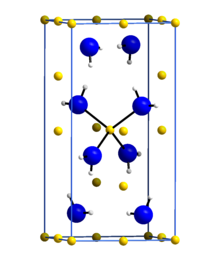
Lithium amide or lithium azanide is an inorganic compound with the chemical formula LiNH2. It is a white solid with a tetragonal crystal structure.[1] Lithium amide can be made by treating lithium metal with liquid ammonia:[2]
- 2 Li + 2 NH3 → 2 LiNH2 + H2
- ^ David, William I. F.; Jones, Martin O.; Gregory, Duncan H.; Jewell, Catherine M.; Johnson, Simon R.; Walton, Allan; Edwards, Peter P. (2007-02-01). "A Mechanism for Non-stoichiometry in the Lithium Amide/Lithium Imide Hydrogen Storage Reaction". Journal of the American Chemical Society. 129 (6): 1594–1601. doi:10.1021/ja066016s. ISSN 0002-7863. PMID 17243680.
- ^ P. W. Schenk (1963). "Lithium amide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY, NY: Academic Press. p. 454.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
