
Back هوموسيرين Arabic هوموسرین AZB Homoserina Catalan Homoserin German Homoserina Spanish هوموسرین Persian Homoseriini Finnish Homosérine French Homoserina Galician ホモセリン Japanese
| |

| |
| Names | |
|---|---|
| IUPAC name
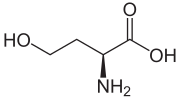
(S)-2-Amino-4-hydroxybutanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.538 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H9NO3 | |
| Molar mass | 119.12 g/mol |
| Melting point | 203 °C (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homoserine (also called isothreonine) is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2OH. L-Homoserine is not one of the common amino acids encoded by DNA. It differs from the proteinogenic amino acid serine by insertion of an additional -CH2- unit into the backbone. Homoserine, or its lactone form, is the product of a cyanogen bromide cleavage of a peptide by degradation of methionine.
Homoserine is an intermediate in the biosynthesis of three essential amino acids: methionine, threonine (an isomer of homoserine), and isoleucine.[1] Its complete biosynthetic pathway includes glycolysis, the tricarboxylic acid (TCA) or citric acid cycle (Krebs cycle), and the aspartate metabolic pathway. It forms by two reductions of aspartic acid via the intermediacy of aspartate semialdehyde.[2] Specifically, the enzyme homoserine dehydrogenase, in association with NADPH, catalyzes a reversible reaction that interconverts L-aspartate-4-semialdehyde to L-homoserine. Then, two other enzymes, homoserine kinase and homoserine O-succinyltransferase use homoserine as a substrate and produce phosphohomoserine and O-succinyl homoserine respectively.[3]
- ^ Tanaka M, Kishi T, Kinoshita S (September 1961). "Studies on the Synthesis of l -Amino Acids: Part III. A Synthesis of l -Homoserine from l -Aspartic Acid". Agricultural and Biological Chemistry. 25 (9): 678–679. doi:10.1080/00021369.1961.10857862. ISSN 0002-1369.
- ^ Berg, J. M.; Stryer, L. et al. (2002), Biochemistry. W.H. Freeman. ISBN 0-7167-4684-0
- ^ Liu P, Zhang B, Yao ZH, Liu ZQ, Zheng YG (October 2020). Zhou NY (ed.). "Multiplex Design of the Metabolic Network for Production of l-Homoserine in Escherichia coli". Applied and Environmental Microbiology. 86 (20). Bibcode:2020ApEnM..86E1477L. doi:10.1128/AEM.01477-20. PMC 7531971. PMID 32801175.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search