
Back اصطناع لامتناظر Arabic Síntesi asimètrica Catalan Asymetrická syntéza Czech Enantioselektive Synthese German Síntesis asimétrica Spanish Asümmeetriline süntees Estonian Sintesi asimetriko Basque سنتز نامتقارن Persian Asymmetrinen synteesi Finnish Synthèse asymétrique French
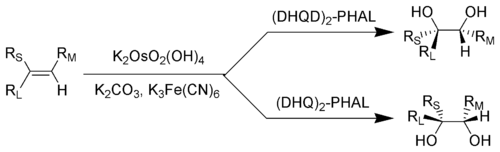
Key: RL = Largest substituent; RM = Medium-sized substituent; RS = Smallest substituent

Enantioselective synthesis, also called asymmetric synthesis,[1] is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal amounts."[2]
Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers.
Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.
- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "asymmetric synthesis". doi:10.1351/goldbook.A00484
- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "stereoselective synthesis". doi:10.1351/goldbook.S05990
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search