
Back Peuja ACE Boraks Afrikaans بورق Arabic بوره AZB Боракс Bulgarian বোরাক্স Bengali/Bangla Boraks BS Tetraboritan sodný Czech Бура (япалалăх) CV Boraks Danish
 | |
 | |
| Names | |
|---|---|
| IUPAC name
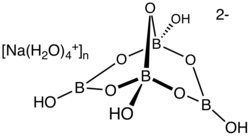
disodium;3,7-dioxido-2,4,6,8,9-pentaoxa-1,3,5,7-tetraborabicyclo[3.3.1]nonane;decahydrate[1]
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number | |
| E number | E285 (preservatives) |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na2B4O5(OH)4·8H2O | |
| Molar mass | 381.36 g·mol−1 |
| Appearance | White or colorless crystalline solid |
| Density | 1.73 g/cm3 (decahydrate, solid)[2] |
| Melting point | 743 °C (1,369 °F; 1,016 K) (anhydrous)[2] 75 °C (decahydrate, decomposes)[2] |
| Boiling point | 1,575 °C (2,867 °F; 1,848 K) (anhydrous)[2] |
| 31.7 g/L [2] | |
| −85.0·10−6 cm3/mol (anhydrous)[2]: p.4.135 | |
Refractive index (nD)
|
n1=1.447, n2=1.469, n3=1.472 (decahydrate)[2]: p.4.139 |
| Structure[3] | |
| Monoclinic, mS92, No. 15 | |
| C2/c | |
| 2/m | |
a = 1.1885 nm, b = 1.0654 nm, c = 1.2206 nm α = 90°, β = 106.623°°, γ = 90°
| |
Lattice volume (V)
|
1.4810 nm3 |
Formula units (Z)
|
4 |
| Pharmacology | |
| S01AX07 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| H360 | |
| P201, P308+P313 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[4] |
REL (Recommended)
|
TWA 1 mg/m3 (anhydrous and pentahydrate)[4][5] TWA 5 mg/m3 (decahydrate)[6] |
IDLH (Immediate danger)
|
N.D.[4] |
| Related compounds | |
Other anions
|
Sodium aluminate |
Other cations
|
Lithium tetraborate |
Related compounds
|
Boric acid, sodium perborate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Borax (also referred to as sodium borate, tincal (/ˈtɪŋkəl/) and tincar (/ˈtɪŋkər/)) is a salt (ionic compound) normally encountered as a hydrated borate of sodium, with the chemical formula Na2H20B4O17.[1][a] Borax mineral is a crystalline borate mineral that occurs in only a few places worldwide in quantities that enable it to be mined economically.
Borax can be dehydrated by heating into other forms with less water of hydration. The anhydrous form of borax can also be obtained from the decahydrate or other hydrates by heating and then grinding the resulting glasslike solid it into a powder. It is a white crystalline solid that dissolves in water to make a basic solution due to the tetraborate anion.
Borax is commonly available in powder or granular form and has many industrial and household uses, including as a pesticide, as a metal soldering flux, as a component of glass, enamel, and pottery glazes, for tanning of skins and hides, for artificial aging of wood, as a preservative against wood fungus, as a food additive, and as a pharmaceutic alkalizer. In chemical laboratories it is used as a buffering agent.[1][8]
The terms tincal and tincar refer to the naturally-occurring borax historically mined from dry lake beds in various parts of Asia.[9]
- ^ a b c d Cite error: The named reference
NIMH.boraxwas invoked but never defined (see the help page). - ^ a b c d e f g Cite error: The named reference
haynes2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
levy1978was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
PGCH57was invoked but never defined (see the help page). - ^ Cite error: The named reference
PGCH59was invoked but never defined (see the help page). - ^ Cite error: The named reference
PGCH58was invoked but never defined (see the help page). - ^ Cite error: The named reference
NFPA2016was invoked but never defined (see the help page). - ^ Cite error: The named reference
CompToxwas invoked but never defined (see the help page). - ^ Cite error: The named reference
amoz2004was invoked but never defined (see the help page).
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
